What is an Atom? [The Basic Units of Matter]
Table of Contents
The atom is the smallest particle into which an element can be divided without losing its chemical properties. Atoms are microscopic. The word atom, of Greek origin, means “indivisible”. Today the subatomic particles are already known (they are the ones that make up an atom) and it is known how an atom can be divided. Let’s see then what an atom is, its structure and how it is composed.

The History of the Atom: How it all began
Have you ever wondered what everything we see is made of? Ancient scientists also asked that question.
Democritus, a Greek philosopher was the first person to use the term atom (atomos means indivisible). He stated that in order to know the composition of matter, we should successively cut it in half.
On our planet we find objects made of different materials. But… what are these materials made of? What is matter?
In the 1700s, scientists discovered that everything around us was made of matter. Matter is “that” which makes up the objects we see every day and can appear in various forms (solid, liquid, gas).
► Let’s Split an Apple down to the Atom

Imagine a sheet of paper that we tear over and over again, obtaining smaller and smaller pieces with each cut. By constantly dividing a body of any material, we will obtain a smaller and smaller piece until a minimum INDIVISIBLE portion. Democritus called this particle an atom.
All matter is made of atoms. Atoms are the smallest, most basic and unbreakable building blocks of matter. First, scientists claimed that the atoms that make up the same object are exactly the same as each other, like identical twins. However, atoms can also be combined with other types of atoms to make a new solid, liquid, or gas. For example, when you see bubbling, burning, color changes, or a new smell, that is simply atoms rearranging each other to form a new substance.
Difference between Atoms and Elements
Atoms are the general term used to describe pieces of matter. You have trillions upon trillions of atoms in your body. However, you may only find around 40 items. You will find billions of hydrogen atoms (H), billions of oxygen atoms (O) and many others. All atoms are made of the same basic building blocks, but they are organized in different ways to make unique elements.
What are Atoms and Molecules: Difference
Molecules are groups of bonded atoms in the same way that words are groups of letters. An A will always be an A no matter what word it is on. A sodium atom (Na) will always be a sodium atom no matter what compound it is in. While atoms have different masses and organization for each element. Electrons, protons, and neutrons make the universe the way it is.
The Atom: From the simplest to the most complex
Smaller particles can work together and create macromolecules. Everything you see or imagine is built by something else. You can start small.

- Particles of matter
- Atoms
- Molecules
- Macromolecules
- Organelles
- Cells
- Organs
- Systems
- Organisms
- Populations
- Ecosystems
- Biomes
- Planets
- Systems with stars
- Galaxies
- The universe
► How to draw an Atom Model
Atomic Structure & Basic Concepts: Protons, Neutrons and Electrons
The atom is made up of 3 (three) subatomic particles (electrons, protons and neutrons).
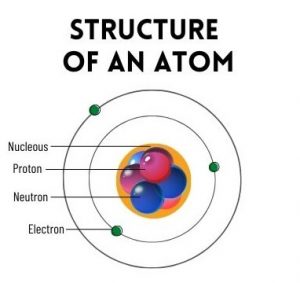
Parts of an Atom
Structurally it is composed of a nucleus composed of one or more protons and typically a similar number of neutrons and one or more electrons orbiting the nucleus. Approximately 99.94% of the mass of the atom is in the nucleus.
Protons have a positive electrical charge, electrons have a negative electrical charge, and neutrons have no electrical charge. If the number of protons and electrons are equal, that atom is electrically neutral.
If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and is called an ion (anion if negative, cation if positive).
Summary: Characteristics of an Atom
Electrons
Electrons are practically massless, negatively charged particles that move around the nucleus. They are attracted to the protons in an atomic nucleus by the electromagnetic force. When there are the same number of electrons and protons in an atom, the atom has a neutral charge. Electrons are attracted to nuclei by the positive charge of the protons. Electrons are much smaller than neutrons and protons. About 1,800 times smaller.
Protons
Protons have mass and are found in the nucleus of the atom, along with the neutrons. They have a positive charge. The hydrogen atom is unique in that it has only one proton and no neutrons in its nucleus.
Neutrons
Neutrons are found in the nucleus of the atom, have the same mass as protons, and have no charge.
How do Atoms differ from each other?
- The number of protons in the nucleus defines which chemical element the atom belongs to.
- The number of neutrons defines the isotope of the element.
- The number of electrons influences the magnetic properties of an atom.
Atoms can join one or more other atoms and form molecules. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature, a subject studied by chemistry.
Chemical Elements and Chemical Symbols
A chemical element is a basic substance that cannot be broken down into simpler substances. Chemical elements are the building blocks of all matter.
More than 118 elements are known, some have been found in nature. Others have been created by scientists artificially.
Some elements are very common and necessary, such as carbon, oxygen, nitrogen or hydrogen. They have been ordered according to their physical and chemical properties, giving rise to the so-called “Periodic Table“.
Chemical Symbols
They are the different abbreviated signs used to identify chemical elements and compounds instead of their full names. The symbol that can be the first letter of your name. For example, H is the symbol for Hydrogen and O for Oxygen. Instead, others are represented with two letters. For example, Cl is the symbol for chlorine, Na is for sodium.
Some frequent elements and their symbols are: carbon, C; oxygen, O; nitrogen, N; hydrogen, H; chlorine, Cl; sulfur, S; magnesium, Mg; aluminum, Al; copper, Cu; argon, Ar; gold, Au; iron, Faith; silver, Ag.
Periodic Table of Elements Easy to Read
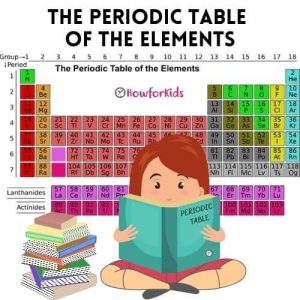
Chemical elements are the basic substances that make up all matter. Dmitry Mendeleyev, a Russian chemist, developed the first periodic table in 1869. It is a system for ordering these chemical elements.
Atomic Number & Atomic Mass with examples for Kids
For example: Each chemical element has an atomic number (it comes from the number of protons it contains in each atom of the element).
Atomic mass is determined by the number of protons and neutrons in the nucleus. Electrons are not taken into account in the atomic mass because they have hardly any mass. Examples:
Oxygen: Element number 8 in the Periodic Table, with 8 protons and 8 neutrons in the nucleus. Located in position number 8 of the table, with an atomic mass of 16.
Carbon: Element number 6 in the Periodic Table, with 6 protons and 6 neutrons in the nucleus. Located in position number 6 of the table, with an atomic mass of 12.
How to explain the Periodic Table of Elements to kids
The periodic table is arranged in columns and rows.
• Rows: Elements are arranged in order of their atomic number.
• Columns: form groups of elements with similar chemical properties.
Chemical Properties of Atoms
Atoms and Isotopes
The atom of the same element can vary in its number of neutrons in the nucleus. In this case we are talking about an isotope. Most elements have different isotopes, which give atoms different characteristics. The most stable isotope is that of the atom that has the same number of neutrons as protons.
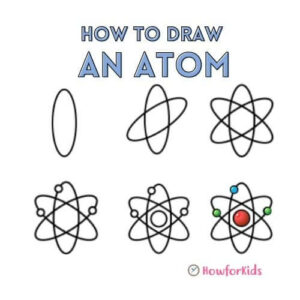
How to Draw an Atom
Eeven smaller particles: Quarks and Neutrinos
Quarks
The quark is a really small particle that makes up the neutrons and protons. Quarks are nearly impossible to detect, and only recently have scientists discovered that they exist. They were discovered in 1964 by Murray Gell-Mann. There are 6 types of quarks: up, down, top, bottom, charm, and strange.
Neutrinos
Neutrinos are formed by nuclear reactions. They are like uncharged electrons and are usually traveling at the speed of light. Trillions and trillions of neutrinos are emitted by the sun every second. Neutrinos pass through most solids, even through humans!
The Atomic Bomb
In a nuclear explosion, the nucleus of the atom (center) is split. The neutrons are released and hit other nuclei, creating a chain reaction. The result is a huge discharge of energy in an explosion of heat, light, and radiation.
How are Atoms divided?
Protons and neutrons are held together in the nucleus at the center of the atom by a very strong force. But this force can be overcome by hitting the nucleus with a neutron, a proton, or any other particle. The nucleus can split and form new atoms. Atoms are split inside nuclear reactors and during nuclear explosions.
What holds Atoms together?
Electrons carry a negative charge and protons a positive charge. The attraction between the two keeps the electrons orbiting. When atoms join together, they share electrons in their outer shells to form chemical bonds.
Carbon Atomic Structure
Carbon Atomic Structure: Diagram
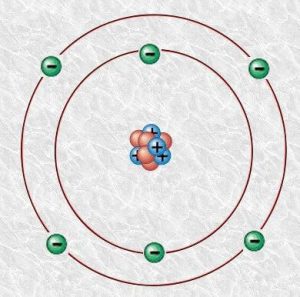
This is the model of a carbon atom. The nucleus of a carbon atom contains six neutrons, six protons. Six electrons orbit the nucleus in two shells.
Summary: Key Concepts
Atoms are made up of parts extremely tiny particles called protons, neutrons, and electrons.
Protons and neutrons are in the center of the atom and form the nucleus.
Electrons revolve around the nucleus.
Protons have a positive charge.
Electrons have a negative charge.
The charge of the proton and the electron are exactly the same size, but opposite.
Neutrons have no charge.
Since opposite charges attract, protons and electrons attract each other.
Read also: The Solar System for Kids
Fun Facts about Atoms
- Why did Democritus in ancient Greece call them atoms? Well, in Greek, atom means: “indivisible”.
- An atom is the smallest particle of an element with the same chemical properties.
- Particles that are smaller than the atom are called subatomic particles.
- At the center of the atom is the nucleus, which is made up of subatomic particles called protons and neutrons. Orbiting at incredible speeds are the electrons.
- By combining these atoms in different ways we can create anything in the universe.
- When atoms combine they form molecules.
- Although protons and neutrons make up the nucleus, most of the nucleus is empty space.
- The most abundant atom is hydrogen. Almost 74% of the atoms in the milky way are hydrogen atoms.
- Atoms move faster in gas form (rather than in liquid or solid form). In solid materials, the atoms are so close to each other that they vibrate, but they are not able to move (there is no space) as water atoms do.
- You have about 7 trillion trillion trillion atoms in your body and you replace 98% of them each year.
