All the objects that surround us are made up of matter. Heat causes changes in matter that are measured by temperature. What is heat? What is the difference between heat and temperature? What is the effect of heat on matter? Keep reading to find out!
What is Matter? Definition for kids
Table of Contents
Matter is the substance from which things are made. Matter is anything that has weight and takes up space. Anything that has volume. The general properties of matter are those that allow us to differentiate some substances from others. These are: mass, volume and temperature.
What is the Difference between Heat and Temperature?
When we describe the objects that surround us, we use words such as: cold, warm, hot, fresh, temperate, among others. Although we sometimes use the words heat and temperature as if they were synonyms, they do not mean the same thing. The difference is that temperature can be measured.
Heat is thermal energy. And temperature is the way to measure heat.
Let’s explain these concepts in more detail with examples. Our body can feel hot or cold through the sense of touch. Nerve endings tell us when an object is hot so we can remove our hand and avoid getting burned. But when it comes to temperature, our sense of touch is limited. For this reason we have thermometers.
Difference Between Heat and Thermal Energy
Heat is a form of energy, called thermal energy. The flow of thermal energy is transferred from one object to another. The more thermal energy an object has, the hotter it is. When a body absorbs heat, its temperature increases. On the other hand, when a body releases heat, its temperature decreases and it cools.
What is temperature?
Temperature is used to measure the amount of thermal energy that objects have. Using temperature we determine how hot an object is. The hotter a material is, the higher its temperature. Temperature increases when an object gains heat and decreases when it loses heat.
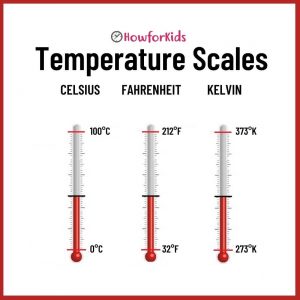
Temperature Scales: Celsius, Fahrenheit, Kelvin
To establish the temperature of an object, a number must be assigned to it. For this, scientists designed graduated temperature scales. There are several temperature scales.
Fahrenheit Scale
The Fahrenheit scale of temperature is the common form of temperature measurement used in the United States. On this scale, 0°C is equivalent to 32°F and 100°C corresponds to 212°F (degrees Fahrenheit).
Celsius Scale
Outside the United States, most of the world uses the Celsius scale to measure temperatures. It indicates that, at sea level, there is a 100-degree difference between cold water (freezing) and boiling water. Water at the point of freezing is 0°C (zero degrees Celsius) and water at its boiling point is 100°C (one hundred degrees Celsius).
Swedish physicist Anders Celsius came up with this scale so it is symbolized with a C for Celsius, after the symbol for degree °. Temperatures below 0°C are indicated with a negative (-) sign. For example -18°C indicates 18 degrees below zero (such as the freezer temperature).
Kelvin Scale
It is used in scientific activity. Here 0°K (zero degrees Kelvin) corresponds to the absence of heat. It is called absolute 0 (zero), which is the lowest temperature that matter can have.
How is temperature measured? Tools Used to Measure Temperature
To measure the temperature we use thermometers. They take advantage of the change in volume of a liquid when it is heated. Liquid column thermometers have a bulb or ampoule filled with liquid (mercury) connected to a thin tube.
How does a Thermometer work?
When the bulb of the thermometer comes into contact with a body that is at a higher temperature, it absorbs heat. The liquid inside the thermometer expands and rises through the capillary (the tube). According to the height that the liquid reaches, it will indicate the temperature on a scale. If you put a thermometer to very high temperatures it can break. If the mercury inside is dropped (it is a very toxic silver liquid) the environment is polluted. Currently, health centers have stopped using mercury thermometers, replaced by a harmless red liquid.
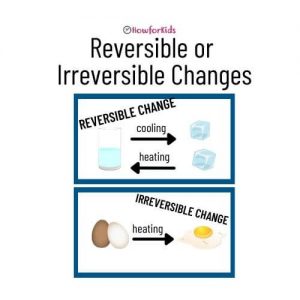
Heat: Reversible and Irreversible Changes
When the normal conditions of the environment change, the temperature rises or falls, the state of the material can change. What happens when materials are exposed to heat? For example, when we heat water it goes from liquid to gaseous (steam), or from solid (ice) to liquid. These changes of state, because they can “go back”, are called reversible changes.
Reversible Changes
Any changes which can be reversed or are a temporary conversion are known as reversible changes. The reactions which are reversible are called reversible reactions. In this reaction, one substance is modified into another form but a new compound is not formed. Processes such as: melting, boiling, evaporation, freezing, condensation and dissolution are reversible changes.
Irreversible Changes
Unlike reversible changes, irreversible changes are permanent. A completely new compound is formed and they cannot be reversed. Heating, burning, mixing and spraying are some of the processes that cause irreversible changes. A common observable example is the cooking of raw egg which cannot be reverted to its original form. Another example: in the case of combustion of wood or burning paper, the material burns and is not recovered.
What are the Effects of Heat on Matter?
Not all objects change their temperature with the same ease. In some materials, the temperature varies rapidly when they gain or lose heat, while in others, the temperature varies more slowly. The speed of temperature variation depends on the type of material and the amount of material with which the objects are made. The higher the amount, the longer it takes for the object to heat up.
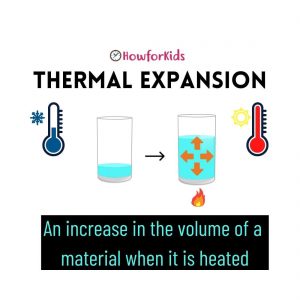
Heat causes changes in materials. Expansion is one of the effects of heat on materials. It is the increase in size that a material undergoes when heated.
Hot Objects Expand
Air, when heated, increases its volume. Increasing or decreasing the temperature of an object can cause changes in its properties.
Thermal Expansion
It is the phenomenon by which the volume of materials increases with increasing temperature. If an object is heated, its atoms vibrate faster and spread out causing the object to expand. This is known as thermal expansion. When it cools down, the atoms slow down and the object shrinks.
Thermal Contraction
With lowering temperatures, objects shrink. This happens because the space occupied by materials (solid, liquid, gas) depends on their temperature. At a higher temperature, the volume they occupy is greater. As the temperature rises, the particles that make up that object become more agitated. They acquire energy, their routes are longer so they take up more space. Otherwise, if the temperature drops, they move less and take up less space.
There are cases, such as water, in which it does not contract when its temperature decreases, but instead increases. In the freezer, water at 0°C expands its volume (and sometimes, if we leave a plastic bottle in the freezer, it breaks).
The three States of Matter
There are three main states that matter can be in: solid, liquid, and gas. Each one depends on the proximity of its particles and their ability to move.
Solid: Characteristics
Solid bodies have their own shape and occupy a certain volume. In solids the particles are very close and orderly, they hardly have room to move, they just vibrate. Solids cannot be compressed, if they are pressed they do not change their shape. Example of solids: water in the form of ice, hail.
Liquid: Characteristics
Liquids do not have their own shape, but take the shape of the container that contains them. They have their own volume because they occupy a limited space. Liquid particles are disordered and can move past each other. Liquids can be compressed more than a solid, when we exert pressure. Example of liquids: water (in its liquid form), oil, honey are viscous liquids, because they do not flow slowly and slide with greater difficulty.
Gas: Characteristics
Gases are characterized in that they have no volume or shape of their own. They not only take the shape of the container that contains them, but also take up as much space as possible. In gases, the particles that make them up are very far apart, in a disorganized way. They move at high speed in all directions. Gases are much more easily compressed than liquids. Examples of gases: water vapor, air.
Fun Fact: Plasma The Fourth State of Matter
There is a fourth state called plasma. It is the usual state in which matter is found in the universe, the sun, the stars. The material between them is in the state of plasma heated by nuclear fusion. A material passes into the plasma state when it is heated from the gaseous state to high temperatures above 10,000 °C and subjected to pressure
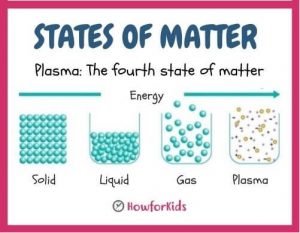
What is Plasma?
Plasma is the fourth state of matter. It is a gas that has been given energy. There comes a point where some electrons are released from the atoms that form the gas, but they continue to coexist, both the freed electrons and the atoms, converted into ions.
When a material or any type of matter is in this state, it is said to be in a state of Plasma. Plasma consists of a collection of electrons and ions that move freely within matter. It is an ionized gas, in which its atoms become positive ions due to the liberation of some of its electrons.
Heat produces changes in materials
Matter is made up of particles. Temperature is related to the speed at which the particles that make up the material move. When an object receives heat, the particles begin to move faster and its temperature increases. On the contrary, if the material releases heat, the movement of the particles is slower, and the temperature is lower.
More Effects of Heat on Matter
For the more advanced…
We are surrounded by matter. Everything around us, including ourselves, is matter. Although all matter is different, there are a series of characteristics that allow us to classify it according to its state of aggregation. That is, how its molecules are held together. There are several criteria:
- Volume: It is the place that a body occupies in space, it can be constant, expand or contract.
- Shape: The shape is taken into consideration insofar as the matter in question can take the shape of the container that contains it, filling everything, or else keep its own shape.
- Compressibility: It is the ability of a body to be compressed, to occupy less volume.
- Molecular cohesion: Refers to the force with which the molecules that make up matter join together. These bonds can be strong or weak.
What is Thermal Equilibrium: Definition for Kids
When two bodies at different temperatures are brought into contact, the hotter one gets colder and the cooler one gets hotter. Heat transfer occurs. However, this heat transfer has a limit.
As time passes, the temperatures of both objects tend to equalize at an intermediate value. When both reach the same temperature we say that they reached thermal equilibrium. Heat transfer is always from the hotter object to the lower temperature object. Some materials conduct heat better than others. Solids are better conductors than liquids and liquids better than gases.
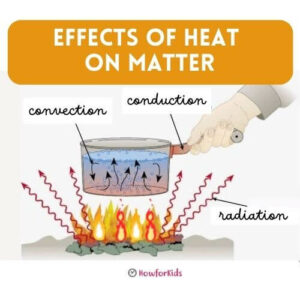
The three types of heat transfer: Conduction, Convection and Radiation
Heat is an energy that is transmitted from one body to another. It doesn’t always do it the same way. There are three different types of mechanisms.
Heat Transfer Mechanisms
Conduction
The transfer of heat through materials, from the hotter object to the cooler object, is called conduction. In conduction, heat transport is between two objects that are in contact or between different parts of the same object. For example, when we place a spoon in a cup of hot liquid, the part that is submerged transfers the heat to the part that is outside. Heat conduction continues until both objects reach the same temperature. They are said to have reached thermal equilibrium.
Best thermal conductors and thermal insulators
The particles of a body that are exposed to the heat source intensify their movement and transmit it to the “neighboring” particles. The movement is transmitted from particle to particle, until it affects all of them. Some materials favor heat transfer more than others, we say that they are “good conductors”. This is the case of metals, because this phenomenon occurs at a higher speed than in others. While other materials are “bad thermal conductors”. For example, plastic, wood or air, have poor conduction capacity. They are called thermal insulators. For example, a frying pan has a plastic or wooden handle so that it does not burn when you grab it.
Convection
Convection is the form of heat transfer that occurs in liquid materials and gases. When air heats up, it expands and rises. For example, when we heat water in a saucepan, the bottom liquid rises and the cooler surface water sinks, heats up, and rises again. Like all other materials, fluids expand when heated. This causes the masses of air or hot water to rise, and those that are at a lower temperature, to descend. The latter, when in contact with the heat source, dilate and rise again. Thus, a circulation of air or water is produced, which is called a convection current.
Radiation
It is a transfer of heat between materials that are not in contact. For example, the heat from the sun’s rays passes through the atmosphere and reaches the surface of Planet Earth. If we bring our hand close to a lamp (without touching it) we feel the heat it gives off, by radiation.
What’s the Difference Between Conduction, Convection and Radiation?
Matter Changing State
Some substances can change from one state to another if they gain or lose heat.
- Melting/ Fusion: Solid to a Liquid.
- Freezing: Liquid to a Solid.
- Vaporization/ Boiling: Liquid to a Gas.
- Condensation: Gas to a Liquid.
- Sublimation: Solid to a Gas.
- Deposition: Gas to a Solid
Temperature and the State of Matter: Solids, Liquids, and Gases
Phase Change: vaporazation, Condensation, Freezing, Melting, Sublimation and Deposition
Examples
If liquid water is cooled below 0°C, it turns into ice which is solid, this is called solidification.
Another example: Making caramel. When sugar is placed in a pudding pan and melted into a brown liquid substance. Once poured into a mould, it is allowed to cool and harden to obtain a caramel. For a liquid to become a solid, it must give off heat.
What is the Melting Point?
When a solid is heated enough it can change to a liquid state by melting. When ice is heated, it melts and turns back into water. The melting point is the temperature at which the transition from the solid to the liquid state occurs, at normal atmospheric pressure.
Matter goes from solid state to liquid state, it melts. When a roast is cooked on the grill, the fat in the meat is lost by melting and dripping.
Other examples: Melt candle, melt cheese.
When water is heated to boiling, it turns into a gaseous state and evaporates by vaporization.
Another example, the puddles that form after the rain, which evaporate once the sun rises, the perspiration on the skin when we exercise, disappears due to progressive evaporation, a glass of alcohol placed at room temperature evaporates.
What is Condensation?
If the vapor cools down it can become liquid water again. The change from gas to liquid is called condensation. For example, when soda cans are very cold and left at room temperature, small “droplets” of water form on the surface. The morning dew that is produced by the decrease in ambient temperature during the early morning allows the condensation of water vapor in the atmosphere on exposed surfaces.
Changes of State of Matter: Examples of each
The change from solid to gas is called sublimation. It is the example of dry ice. Carbon dioxide can be first liquefied and then frozen to make dry ice. And this, at room temperature, recovers its original gaseous form. Naphthalene is used as a clothing preservative to repel moths. Their typical white “balls” disappear by themselves, as they go from the solid to the gaseous state.